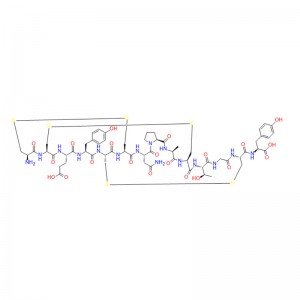
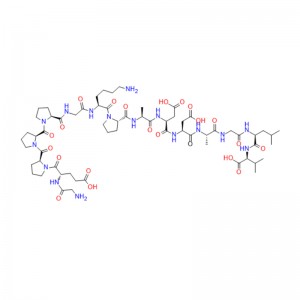
Linaclotide is a cyclic peptide that consists of 14 amino acids, three of which are cysteines that form disulfide bonds. Linaclotide is structurally related to the endogenous peptides guanylin and uroguanylin, which are natural ligands of the guanylate cyclase C (GC-C) receptor. The GC-C receptor is expressed on the luminal surface of the intestinal epithelial cells, where it regulates fluid secretion and intestinal motility. Linaclotide binds to the GC-C receptor with high affinity and specificity, and activates it by increasing the intracellular levels of cyclic guanosine monophosphate (cGMP). cGMP is a second messenger that mediates various cellular responses, such as chloride and bicarbonate secretion, smooth muscle relaxation, and pain modulation. Linaclotide acts locally in the gastrointestinal tract, and does not penetrate the blood-brain barrier or affect the central nervous system. Linaclotide also produces an active metabolite, MM-419447, which has similar pharmacological properties to linaclotide. Both linaclotide and its metabolite are resistant to proteolytic degradation by intestinal enzymes, and are mainly eliminated unchanged in the feces (MacDonald et al., Drugs, 2017).
By activating the GC-C receptor, linaclotide increases the secretion of fluid into the intestinal lumen, which softens the stool and facilitates bowel movements. Linaclotide also reduces the visceral hypersensitivity and inflammation that are associated with irritable bowel syndrome (IBS) and other gastrointestinal disorders. Linaclotide modulates the activity of the enteric nervous system and the colonic nociceptors, which are sensory neurons that transmit pain signals from the gut to the brain. Linaclotide decreases the expression of pain-related genes, such as substance P and calcitonin gene-related peptide (CGRP), and increases the expression of opioid receptors, which mediate analgesia. Linaclotide also reduces the release of pro-inflammatory cytokines, such as interleukin-1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α), and increases the release of anti-inflammatory cytokines, such as interleukin-10 (IL-10) and transforming growth factor beta (TGF-β). These effects of linaclotide improve the symptoms of constipation and abdominal pain in patients with IBS or chronic constipation (Lembo et al., The American Journal of Gastroenterology, 2018).
Linaclotide has been shown to be effective and well-tolerated in several clinical trials involving patients with CC or IBS-C. In these trials, linaclotide improved bowel habits, such as stool frequency, consistency, and completeness; reduced abdominal pain and discomfort; and enhanced quality of life and patient satisfaction. Linaclotide also demonstrated a favorable safety profile, with diarrhea being the most common adverse event. The incidence of diarrhea was dose-dependent and usually mild to moderate in severity. Other adverse events were generally similar to placebo or low in frequency. No serious adverse events or deaths were attributed to linaclotide treatment (Rao et al., Clinical Gastroenterology and Hepatology, 2015).



Linaclotide is a novel and effective medication for patients with CC and IBS-C who have not responded well to conventional therapies. It works by mimicking the action of endogenous peptides that regulate intestinal function and sensation. Linaclotide can improve bowel habits, reduce abdominal pain, and enhance quality of life for these patients.
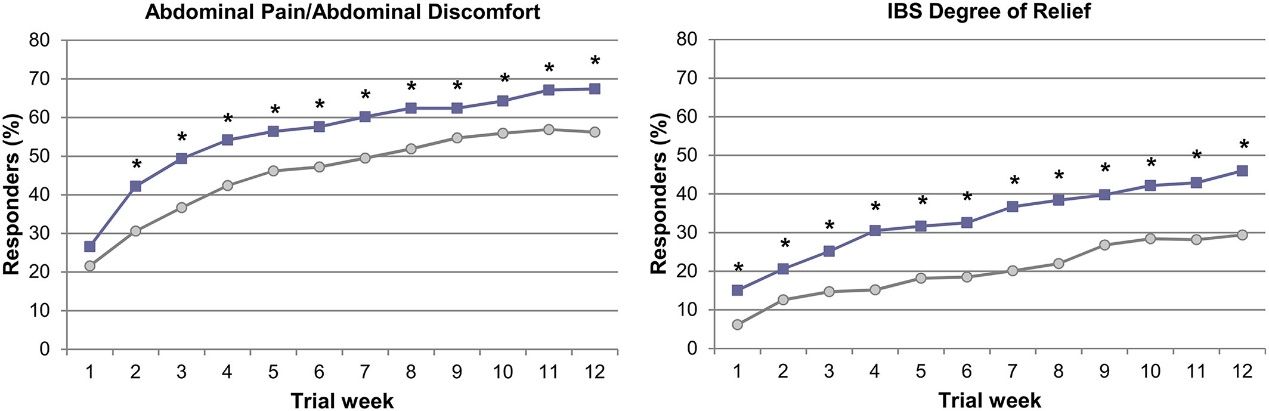
Figure 1. Abdominal pain/abdominal discomfort and IBS degree of relief weekly responders over the 12-week. ?, placebo;?, linaclotide 290?μg.?
(Yang, Y., Fang, J., Guo, X., Dai, N., Shen, X., Yang, Y., Sun, J., Bhandari, B. R., Reasner, D. S., Cronin, J. A., Currie, M. G., Johnston, J. M., Zeng, P., Montreewasuwat, N., Chen, G. Z., and Lim, S. (2018) Linaclotide in irritable bowel syndrome with constipation: A Phase 3 randomized trial in China and other regions. Journal of Gastroenterology and Hepatology, 33: 980–989. doi: 10.1111/jgh.14086.)
We are a polypeptide manufacturer in China, with several years of mature experience in polypeptide production. Hangzhou Taijia Biotech Co., Ltd. is a professional polypeptide raw material manufacturer, which can provide tens of thousands of polypeptide raw materials and can also be customized according to needs. The quality of polypeptide products is excellent, and the purity can reach 98%, which has been recognized by users all over the world.Welcome to consult us.